USP <795> and <797> exist to reduce medication risks that include contamination, infection, or incorrect dosing when compounding both nonsterile and sterile preparations. These standards were last updated in 2014 and 2008 respectively, and the USP revision schedule has spanned much of the last decade. The organization has worked through proposed revisions to both standards to incorporate new scientific understanding, advances in technology, and stakeholder input.
The latest proposed revision was released in September 2021, followed by a lengthy public comment period that ended March 17, 2022. At their April 19, 2022 meeting, the USP Compounding Expert Committee announced the conclusion of comment review and advancement to the next steps to make the revisions official. This marks an important milestone in this long journey and signals that USP is likely to approve and issue the revised standards by some time in 2023.
Based on prior drafts, as well as the introduction of USP <800> in 2016, many pharmacies made investments in facility updates, while deferring other aspects such as training and environmental monitoring until the revisions become official. With a new revision date on the horizon, it is time to dust off those assessments and prepare to implement the new standards.
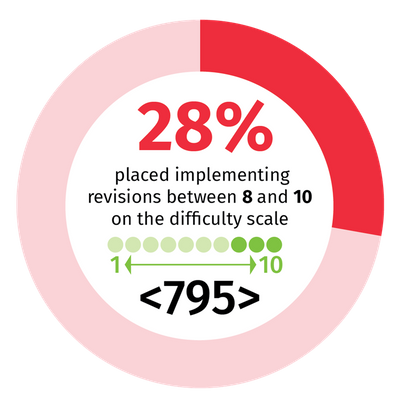
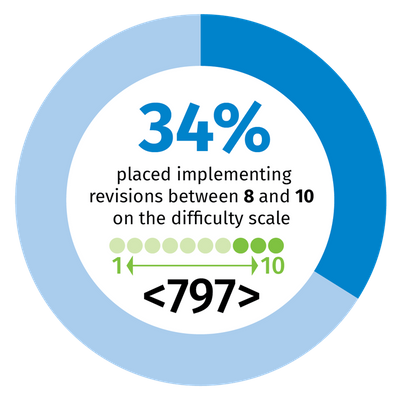
However, making changes to their standard operating procedures requires no small effort. A recent poll of 227 users of Wolters Kluwer’s Simplifi 797 solution – which more than 2,000 pharmacies in the United States use to help ensure compliance with USP <795>, <797> and <800> – uncovered considerable concern. When asked to estimate on a scale from 1-10 the difficulty of implementing the proposed revisions, 28% placed it between 8 and 10 for <795>; for <797>, more than a third of participants, 34%, placed the difficulty between 8 and 10.
 |
 |
Clearly it is time for compounding pharmacies to make sense of what the changes will demand of their particular facility and to begin acting to ensure their operations are fully compliant so they can continue to keep patients safe and medications effective.
Understanding the USP revisions
The proposed revisions to USP <795> and <797> set new standards for nearly every aspect of a compounding pharmacy’s operation, including training and managing personnel, maintaining a facility’s cleanliness, and testing and preparing containers and drug components. In fact, all sections of both chapters were edited in some way during the comment review period. A few items stand out.
For <795> Nonsterile Compounding:
- Training: The revisions emphasize new levels of training that demand staff members demonstrate they know and can perform essential core competencies.
- Cleaning and Sanitizing: Bringing new focus on the nonsterile compounding environment, including new schedules for cleaning and sanitizing “frequently touched surfaces.”
- Beyond-Use-Dates: While in the past, the primary focus for Beyond-Use-Dates (BUDs) has been the chemical and physical properties of the active pharmaceutical ingredient, the proposed revisions also emphasize the container closure system, especially how it interacts with the compounded nonsterile preparation and the potential risks for microbial contamination or a loss of potency. The revisions also focus on measuring the degree of water activity as part of determining a BUD included an expanded table of common dosage forms and their water activity. Additional clarity and criteria for extending BUDs beyond the chapter defaults has also been proposed including stability-indicating assays and antimicrobial effectiveness testing.
As for USP <797>, Sterile Compounding, many of the proposed revisions from the 2019 draft remain but with some additional rationale to support their inclusion.
- Cleaning, sanitizing: Use of sterile products within the Primary Engineering Control (PEC) and use of sporicidal agents will be required.
- Environmental monitoring: no change to prior draft requiring monthly surface sampling in classified environments.
- Beyond-use Dates (BUDs): Category 1 and Category 2 remain with some slight concessions in dating. However a new Category 3 was introduced to provide a framework for the longest BUDs allowed under the USP <797> standards.
- Category 3 sterile preparations can have longer BUDs compared to those for Category 1 and Category 2. Additional criteria for Category 3 compounding include: more frequent cleaning, more frequent environmental monitoring, more frequent competency assessments, sterility testing and a batch size limited to 250 units.
The impact on pharmacies
Each of the revisions will have at least some impact on the people, processes and technology at compounding pharmacies. In a recent article in Pharmacy Purchasing & Products, the authors estimated the resource impact of most of the key proposed changes, both on a pharmacy’s people (labor resources to implement) and on the cost to an organization in actual dollars. In many cases, the impact will not be trivial and, as such, explains the types of concerns uncovered in our survey of Simplifi 797 users.
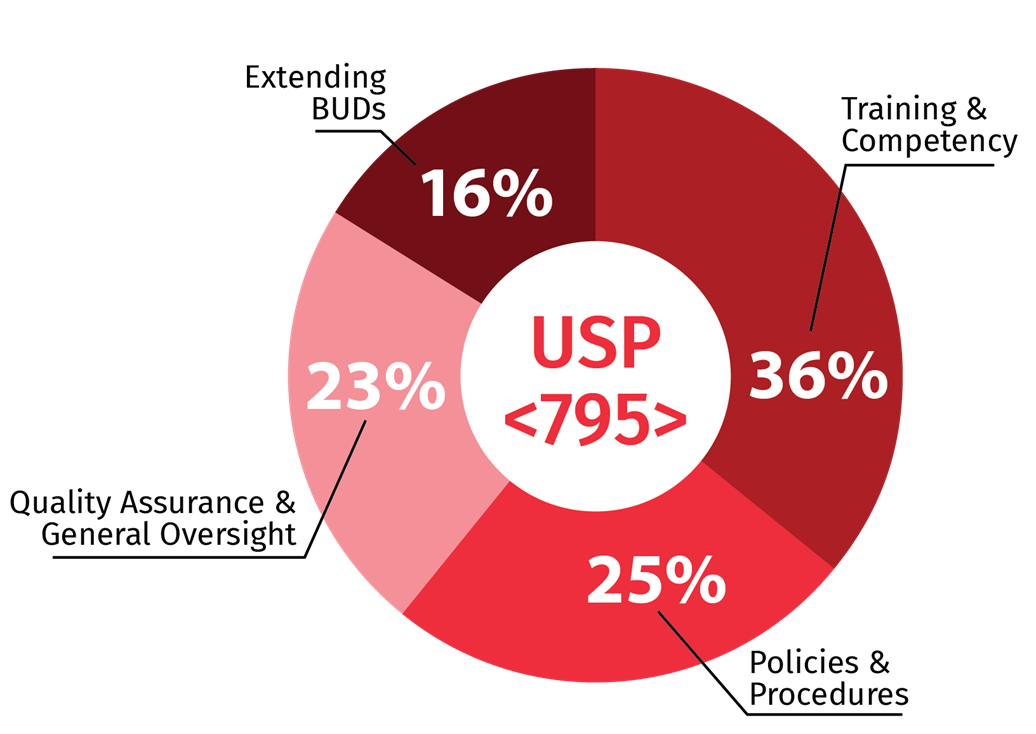
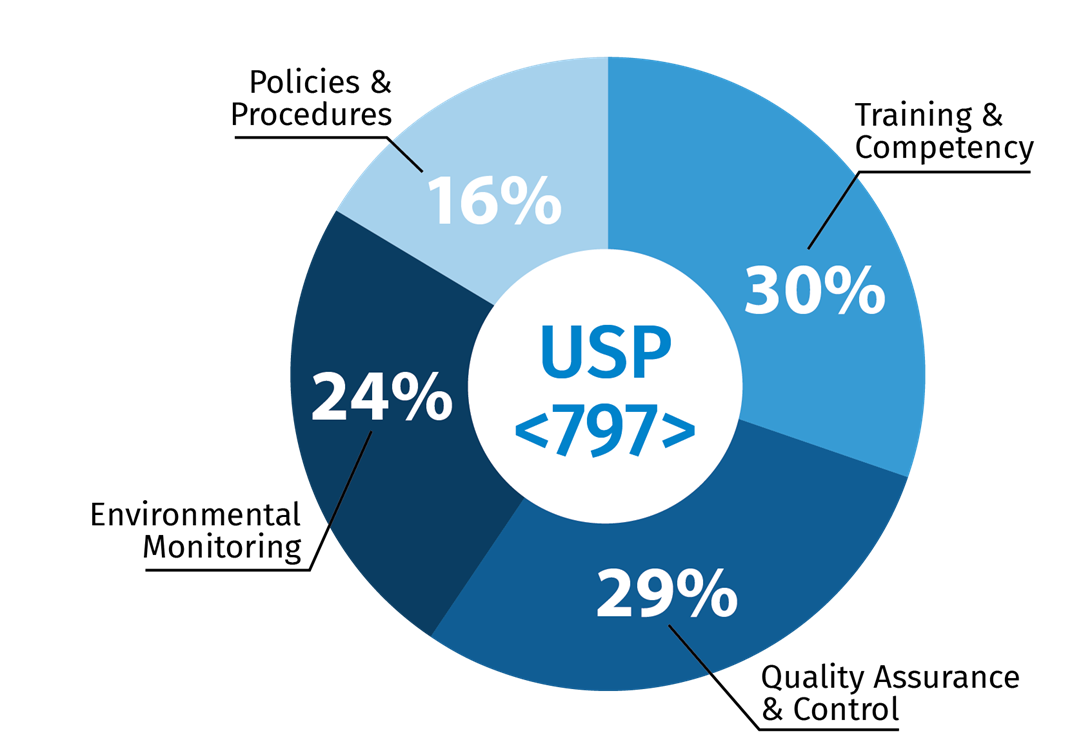
For USP <795>, 36 percent of the respondents listed training and competency as their top concern. This was followed by policies and procedures (25%), quality assurance and general oversight (23%) and implementing a methodology for extending BUDs (16%). For USP <797>, 30% had training and competency as their top concern, followed by quality assurance and control (29%), environmental monitoring (24%) and policies and procedures (16%).
 |
 |
The time to prepare for USP enforcement is now
Many of these actions have been proposed for years and the direction of USP is clear as they strive to raise the minimum standard for higher quality CSPs. Given the potential impact and level of concern, waiting for the USP revision schedule to finalize seems a risky strategy. For one, waiting will not allow proper time to identify new products, formulate new processes and workflows, or identify new vendor partners that may already have contracts pending.Another strategy is testing changes in one compounding area or facility to validate impacts and workflows before a more wide-spread implementation. Adopting proposed practices early also allows you to roll out changes over time rather than implementing many changes all at once, which may ease the mental burden for staff.
For those who don’t have the resources in place, engaging with compounding compliance experts and deploying technology linked closely with the USP standards can significantly ease the burden of change. At Simplifi 797, we are actively updating our content and tools to ease your transition to the latest USP standards, while also enabling pharmacies to tailor their capabilities to their particular workflows. For example, with the flexibility of Simplifi 797, you can stage updated competencies and tasks to be ready for the changes and assign them once the USP revisions become official. In this time of change, having that type of support in place can be invaluable.
