USP <797> (2023) considers additional factors that may introduce risk to the final CSP. These focus primarily on the environment in which the compound is prepared. Examples include air quality and facility design, as well as the frequency of monitoring the environment. Additionally, the sterility of starting ingredients or methods for sterilizing final CSPs must be considered. All these factors increase the risk of microbial contamination in the final CSP. The more controls are put into place, the lower risk and thus longer BUD.
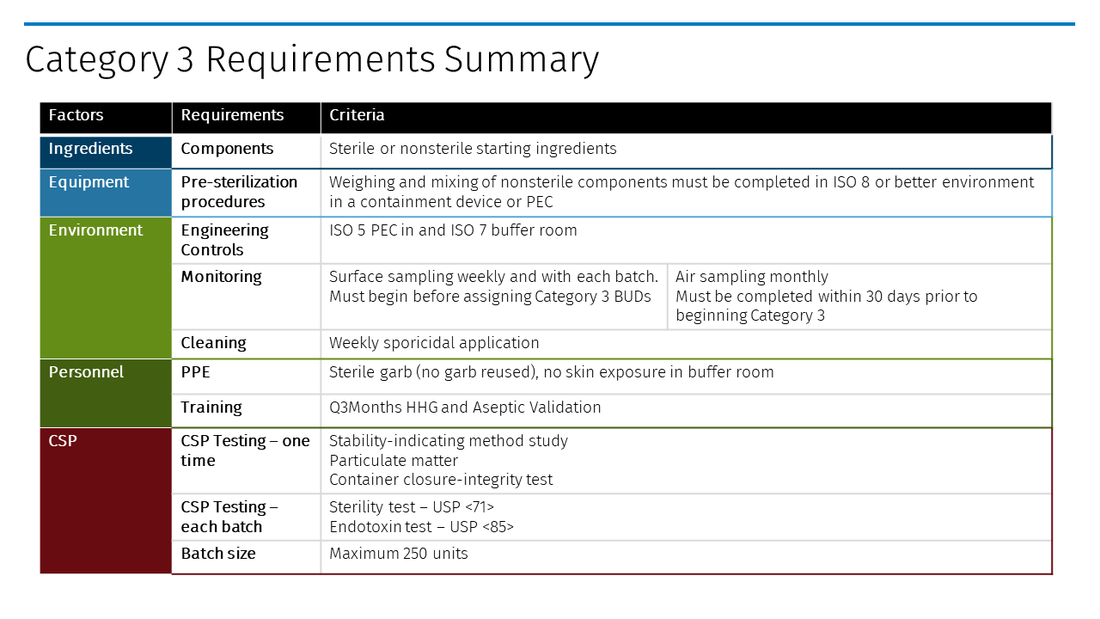
Category 3 CSPs represent the highest risk starting components, requiring more frequent environmental monitoring and personnel competency validation, and the use of sterile personal protective equipment (PPE). Yet those preparing Category 3 CSPs will be rewarded with the longest BUDs allowed in the Chapter.
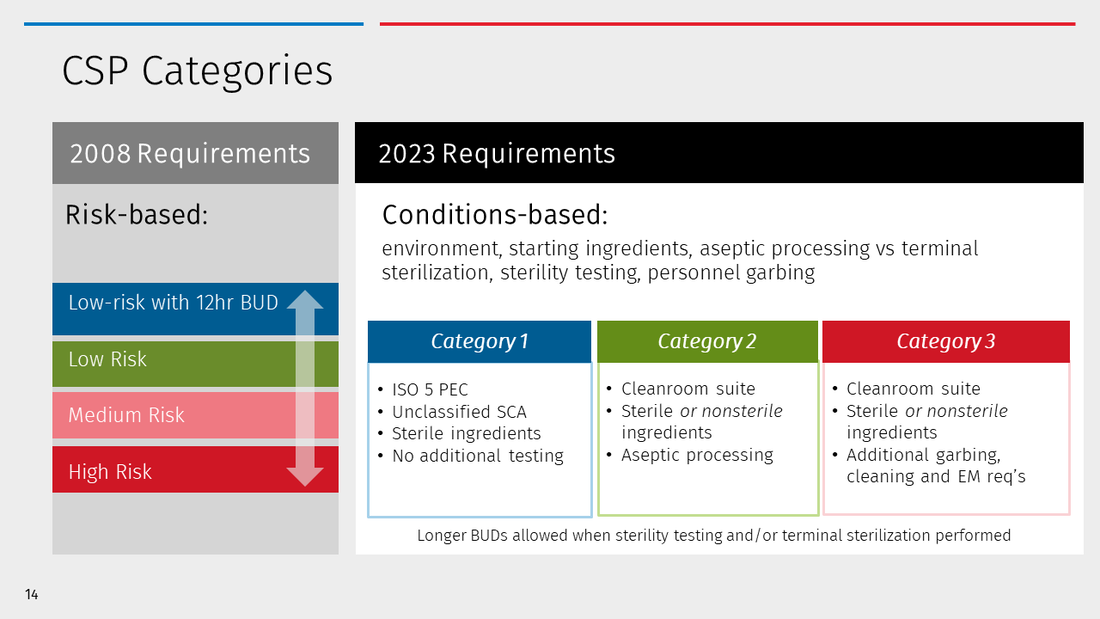
Refer to Table 1 for a comparative summary of current risk levels and new categories for CSPs.
Operational implications of new CSP Categories
Determining CSPs Categories is important because it establishes the beyond-use date (BUD) for each CSP. The BUD is the date and time by which administration of a CSP must begin. In other words, the safe storage limit ensures reasonable sterility of a CSP under certain conditions – without performing a sterility test.
Compound Sterile Preparation Categories
Category 1 CSPs
Category 1 CSPs are prepared in an ISO 5 Primary Engineering Control (PEC) but in an otherwise uncontrolled or unclassified space e.g., a Segregated Compounding Areas (SCA). BUDs are limited to 12 hours at room temperature and 24 hours refrigerated, or less per product stability.
Category 1 BUDs apply when a CSP is prepared under less-than-ideal circumstances, including environmental or procedural conditions, that increase the risk of microbial contamination. Examples include:
- A segregated compounding area (SCA) with inadequate filtration, air pressure, or air exchanges to meet ISO 7 classification criteria.
- Temporary break in the state of control of a classified buffer area, such as power failure resulting in loss of pressure.
Other notable considerations when evaluating the impacts of old risk levels and new categories:
Hazardous Drugs
Per USP <797> (2008), only non-hazardous and radiopharmaceutical CSPs may be prepared in an (SCA) under low-risk with 12-hour BUD conditions. Under the new USP <797> (2023), no limitations on the type of CSPs are mentioned and USP <800> will become compendial applicable. USP <800> describes the criteria for Containment-Segregated Compounding Areas (C-SCAs) that allow for compounding of hazardous drugs with 12-hour (room temp), 24-hour (refrigerated), or less BUD. Minimally, C-SCAs must have:
- At least 12 air changes per hour (ACPH)
- Maintain negative pressure of -0.01 to -0.03 i.w.c. for the room, with an externally vented containment primary engineering control (C-PEC).
Restricted-access barrier systems (RABS)
Examples include Compounding Aseptic Isolators (CAI) and Compounding Aseptic Containment Isolators (CACI) provide an ISO 5 compounding environment. However, if these types of PECs are placed in an SCA, only Category 1 BUDs may be applied. Many organizations have relied on RABS to serve as the sole source of classified air and have completed higher risk level compounding or even anticipatory compounding without additional environmental controls. This is no longer allowed under the new standards. If your facility has a CAI or CACI in an SCA, assess the type of compounding performed and the impacts of decreasing BUDs to a max of 24 hours (refrigerated).
Category 2 CSPs
Category 2 CSPs require a higher level of environmental control including preparation in an ISO 5 PEC and a classified cleanroom suite. Refer to the Chapter for the full criteria of a cleanroom suite including air flow, temperature, humidity, pressure, and design requirements. Most pharmacies with a modern cleanroom design should have the type of environment to support Category 2 BUDs.
BUDs for Category 2 CSPs are determined by the environment as well as the sterility of the starting ingredients and what type of sterilization techniques are used. Examples when prepared in an ISO 5 PEC and a full cleanroom suite:
- Typical or most common scenario: Sterile starting ingredients aseptically prepared with no additional sterility testing results in BUDs of 4 days (room temp), 10 days (refrigerated), 45 days (frozen)
- Maximum BUDs scenario: Terminally sterilized CSPs prepared with additional sterility testing performed and passed results in BUDs of 45 days (room temp), 60 days (refrigerated), and 90 days (frozen).
Category 2 CSPs may be prepared from non-sterile starting ingredients. Longer BUDs are available if sterility testing is performed and passed, with no additional facility monitoring or personnel competency assessments required.